
UK approved COVID-19 vaccine expected in VI in February 2021
According to Minister for Health and Social Development, Hon Carvin Malone (AL), the Government of the Virgin Islands will be collaborating with several agencies in supporting a national programme to make the AstraZeneca vaccine available to residents on a voluntary basis.
Premier and Minister of Finance had first announced that any COVID-19 vaccine made available to the VI by the UK would be given on a voluntary basis.
The first dose of the Oxford-AstraZeneca coronavirus vaccine was given in the UK on January 4, 2021, to dialysis patient Brian Pinker, 82.
The UK government has since ordered 100 million doses of the vaccine, following the roll-out of the Pfizer vaccine, which was the first to be approved.

According to Minister for Health and Social Development, Hon Carvin Malone (AL), the Government of the Virgin Islands will be collaborating with several agencies in supporting a national programme to make the AstraZeneca vaccine available to residents on a voluntary basis.
National Programme
In a statement in the House of Assembly during the Fifth Sitting of the Third Session of the Fourth House of Assembly (HoA) at Save the Seed Energy Centre in Duff’s Bottom, Tortola, on December 31, 2020, Minister for Health and Social Development, Honourable Carvin Malone (AL) said his ministry will work closely with Public Health England, the Caribbean Public Health Agency, CARPHA, the Pan American Health Organisation (PAHO), and the World Health Organisation (WHO) on a national programme to make the vaccine available to residents on a purely voluntary basis.
Honourable Malone said, “Published safety data indicates that this vaccine is well tolerated and has no serious safety events confirmed related to the vaccine. Safety data will continue to be collected and monitored as the vaccine is rolled out world-wide including in the BVI. Work on a national programme to make the vaccine available to residents on a purely voluntary basis will start early in the new year in preparation for when the vaccine is delivered in the Territory.”
The national programme will be led by the Ministry of Health and involve collaboration and communication with a wide cross-section of stakeholders across government, private, and non-government sectors.
The vaccine is expected to be available in the Virgin Islands in February 2021 as the United Kingdom Government plans for deployment of the vaccine throughout the Overseas Territories.

A technician working on the Oxford/AstraZeneca Covid vaccine.
Vaccination an important strategy to end menace of COVID-19- Hon Malone
Honourable Malone explained that effective communication and innovative strategies are crucial to addressing the public’s concerns regarding the vaccine. He said that the Ministry of Health’s objective is to reach out to the community with information and offer vaccine to all targeted populations.
The Minister for Health said, “Vaccination of the world’s population is an important strategy to end the continued menace of COVID-19 to the world. The Territory must not only do its part in this effort, but also for our own sakes to reduce the impact of this pandemic on the Territory, its inhabitants, and our economy.”
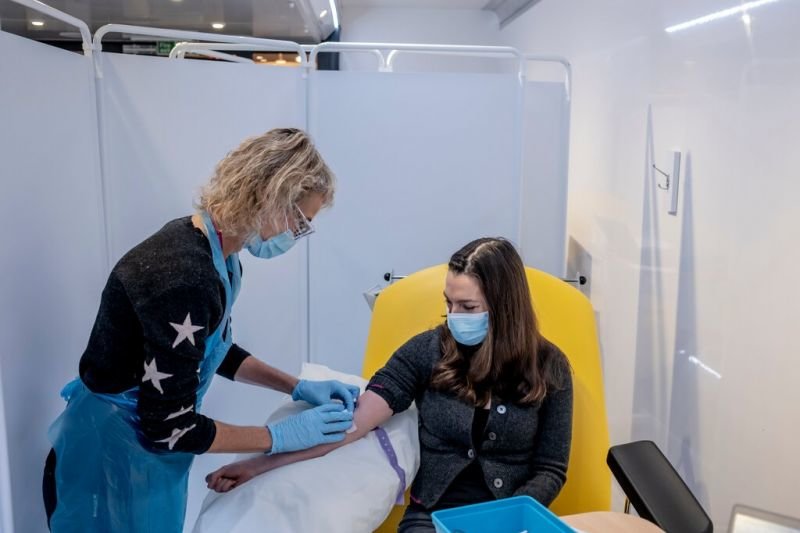
A volunteer participating in the AstraZeneca vaccine trial having blood drawn in Oxford, England, last week.
AstraZeneca vaccine
The UK approval of the Oxford/AstraZeneca vaccine has been hailed by scientists and health experts as game-changing step for tackling the coronavirus in the developing world.
Researchers at the University of Oxford built the vaccine using a kind of virus, called an adenovirus, that typically causes colds in chimpanzees. They genetically altered the virus so that it carried a gene for a coronavirus protein, which would theoretically train a person’s immune system to recognise the real coronavirus.
According to The Telegraph on December 30, 2020, the drug was made with technology and funding deliberately aimed at tackling future pandemics and will cost a fraction of existing jabs. Its ease of transport and storage as well as a worldwide manufacturing effort will bring it within reach of hundreds of millions of people in poorer countries.
“Approval of this vaccine is a turning point for the pandemic,” said Professor Helen Fletcher, at the London School of Hygiene & Tropical Medicine. “It has been deliberately developed to have global impact that includes people living in the most fragile and poorest regions of the world.”
The Oxford/AstraZeneca vaccine can be stored at the temperature of a conventional fridge, while the Pfizer/ BioNTech vaccine approved earlier in December 2020 must be stored at -70C.
African governments are also thought to be keen to use the drug because it was trialled successfully in South Africa and then in Kenya.
Is the AstraZeneca vaccine safe?
According to the New York Times on December 30, 2020, for years, Oxford researchers have been testing their chimpanzee adenovirus vaccine, ChAdOx1, on a number of other diseases including Ebola and Zika.
Although none of those studies have reached the final, so-called Phase 3 trials, they have allowed researchers to examine the safety of the vaccine platform.
The researchers have not found any serious side effects.











