UK Government Unveils Roadmap to End Animal Testing via AI and Human-Tissue Innovations
Science minister sets timelines for replacement of animal tests with organ-on-a-chip, 3D bioprinting and AI-driven methods across regulatory domains
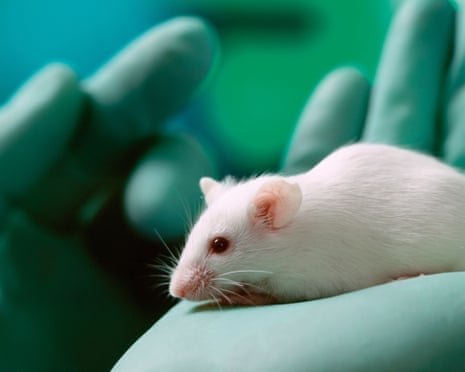
The UK government has launched a new strategy aimed at phasing out certain uses of animals in scientific and regulatory testing by harnessing artificial intelligence and human-tissue alternatives.
Announced by the Science Minister, Lord Patrick Vallance, the roadmap signals a shift towards technologies such as organ-on-a-chip systems, 3D-bioprinted tissues and AI-based molecular safety analysis.
According to the plan, animal testing for skin and eye irritation and skin-sensitisation will cease by the end of 2026. By 2027 all potency testing of botulinum toxin (Botox) on mice is to end, and by 2030 major pharmacokinetic studies involving dogs and non-human primates will be scaled back significantly.
The government emphasises that animal testing will only be eliminated where alternative methods can ensure equivalent or improved safety for humans and the environment.
The roadmap is supported by commitments to fund researchers, streamline regulation and build the necessary infrastructure for non-animal methods.
Technologies such as organ-on-a-chip devices—miniaturised models of human organs using real human cells—and AI systems designed to analyse large datasets of molecular behaviour are central to the strategy.
3D-printed tissues from human cells will offer testing of skin, liver and other organs without relying on live animals.
Lord Vallance said: “Nobody in our country of animal lovers wants to see suffering, and our plan will support work to end animal testing wherever possible and roll out alternatives as soon as it is safe and effective to do so.” The Royal Society for the Prevention of Cruelty to Animals welcomed the strategy, saying it sets “a clear ambition towards eliminating animal use and supports increased access to the infrastructure, collaborations and resources required.”
The document clarifies that the planned reductions apply first to regulatory testing—across cosmetics, chemicals and medicines—while standard animal research in diagnostics or basic science may continue under strict conditions.
The government also indicated that the regulator for animals in science will strengthen its oversight, data monitoring and transparency under reforming regulation announced in October 2024.
With the strategy now published, the UK joins a growing number of jurisdictions prioritising non-animal testing technologies, and positions itself as a potential global leader in “new approach methodologies”.
According to government data, in 2023 there were approximately 2.68 million animal procedures legally recorded in Great Britain, of which roughly nine percent involved regulatory safety testing.
The new plan reflects both ethical and scientific ambitions to reduce animal use while maintaining high standards of human-safety and environmental-protection.
Announced by the Science Minister, Lord Patrick Vallance, the roadmap signals a shift towards technologies such as organ-on-a-chip systems, 3D-bioprinted tissues and AI-based molecular safety analysis.
According to the plan, animal testing for skin and eye irritation and skin-sensitisation will cease by the end of 2026. By 2027 all potency testing of botulinum toxin (Botox) on mice is to end, and by 2030 major pharmacokinetic studies involving dogs and non-human primates will be scaled back significantly.
The government emphasises that animal testing will only be eliminated where alternative methods can ensure equivalent or improved safety for humans and the environment.
The roadmap is supported by commitments to fund researchers, streamline regulation and build the necessary infrastructure for non-animal methods.
Technologies such as organ-on-a-chip devices—miniaturised models of human organs using real human cells—and AI systems designed to analyse large datasets of molecular behaviour are central to the strategy.
3D-printed tissues from human cells will offer testing of skin, liver and other organs without relying on live animals.
Lord Vallance said: “Nobody in our country of animal lovers wants to see suffering, and our plan will support work to end animal testing wherever possible and roll out alternatives as soon as it is safe and effective to do so.” The Royal Society for the Prevention of Cruelty to Animals welcomed the strategy, saying it sets “a clear ambition towards eliminating animal use and supports increased access to the infrastructure, collaborations and resources required.”
The document clarifies that the planned reductions apply first to regulatory testing—across cosmetics, chemicals and medicines—while standard animal research in diagnostics or basic science may continue under strict conditions.
The government also indicated that the regulator for animals in science will strengthen its oversight, data monitoring and transparency under reforming regulation announced in October 2024.
With the strategy now published, the UK joins a growing number of jurisdictions prioritising non-animal testing technologies, and positions itself as a potential global leader in “new approach methodologies”.
According to government data, in 2023 there were approximately 2.68 million animal procedures legally recorded in Great Britain, of which roughly nine percent involved regulatory safety testing.
The new plan reflects both ethical and scientific ambitions to reduce animal use while maintaining high standards of human-safety and environmental-protection.